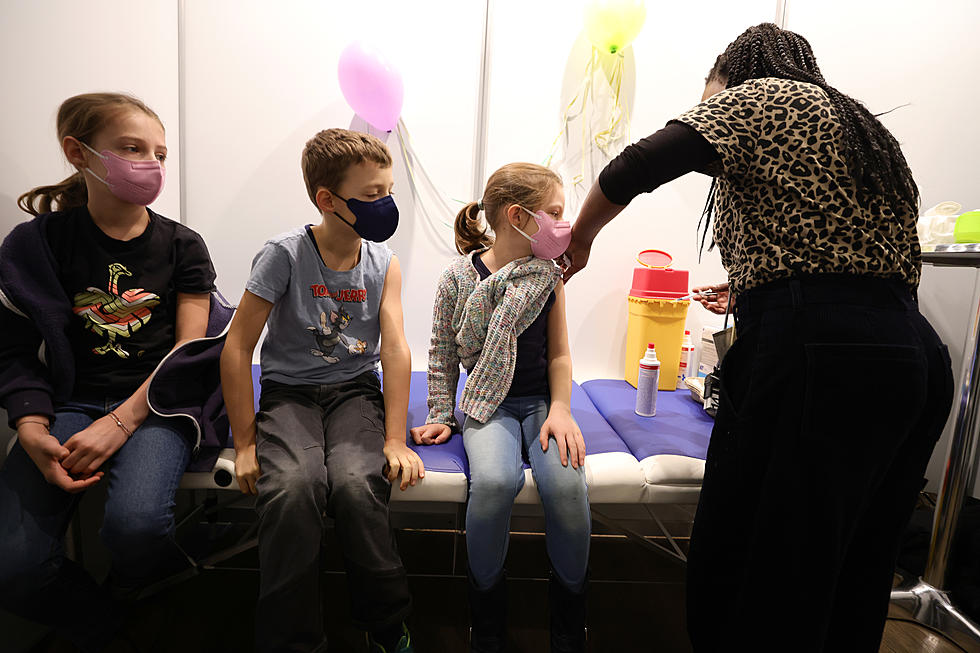
FDA Approves Pfizer Boosters For Teens 12 And Up
Monday it was announced by the FDA that they have approved a Pfizer booster for teenagers 12 and up for extra protection against the Omicron variant. According to reports, COVID-19 boosters have previously been authorized for everyone 16 and older. However, with the rate of Omicron infections occurring around the globe, and hospitalization in kids on the rise (mostly unvaccinated) a third child-size dose is warranted.
The FDA is expanding COVID-19 boosters for children as young as 12 to confront the surge, with an extra Pfizer shot 5 months after their last dose instead of 6 months. The evidence is indisputable, that vaccines offer the strongest protection to prevent serious illness from any COVID-19 variant. CDC and state health officials across the nation are urging all Americans who are eligible to get the vaccine or a booster due so.
The good news is that children tend to suffer less serious illnesses from COVID-19 than adults. But the Omicron wave has seen a significant rise in kids, mostly unvaccinated, being hospitalized nonetheless. So far, the Pfizer/BioNTech vaccine is the only U.S. option for children of any age. According to the CDC to date, an estimated 13.5 million teens in the U.S. ages 12 to 17 have received two shots.
In addition, the FDA announced Monday that younger children with severely weakened immune systems will also be allowed a third dose 28 days after their second. This by the way is the same third-dose time frame already recommended for immune-compromised teens and adults. Pfizer clinical trials are already underway for an even smaller vaccine dose that can be administered to kids 5 and younger.
Inside Amazon: A Detailed History of America's Biggest Online Retailer
More From 107 JAMZ









